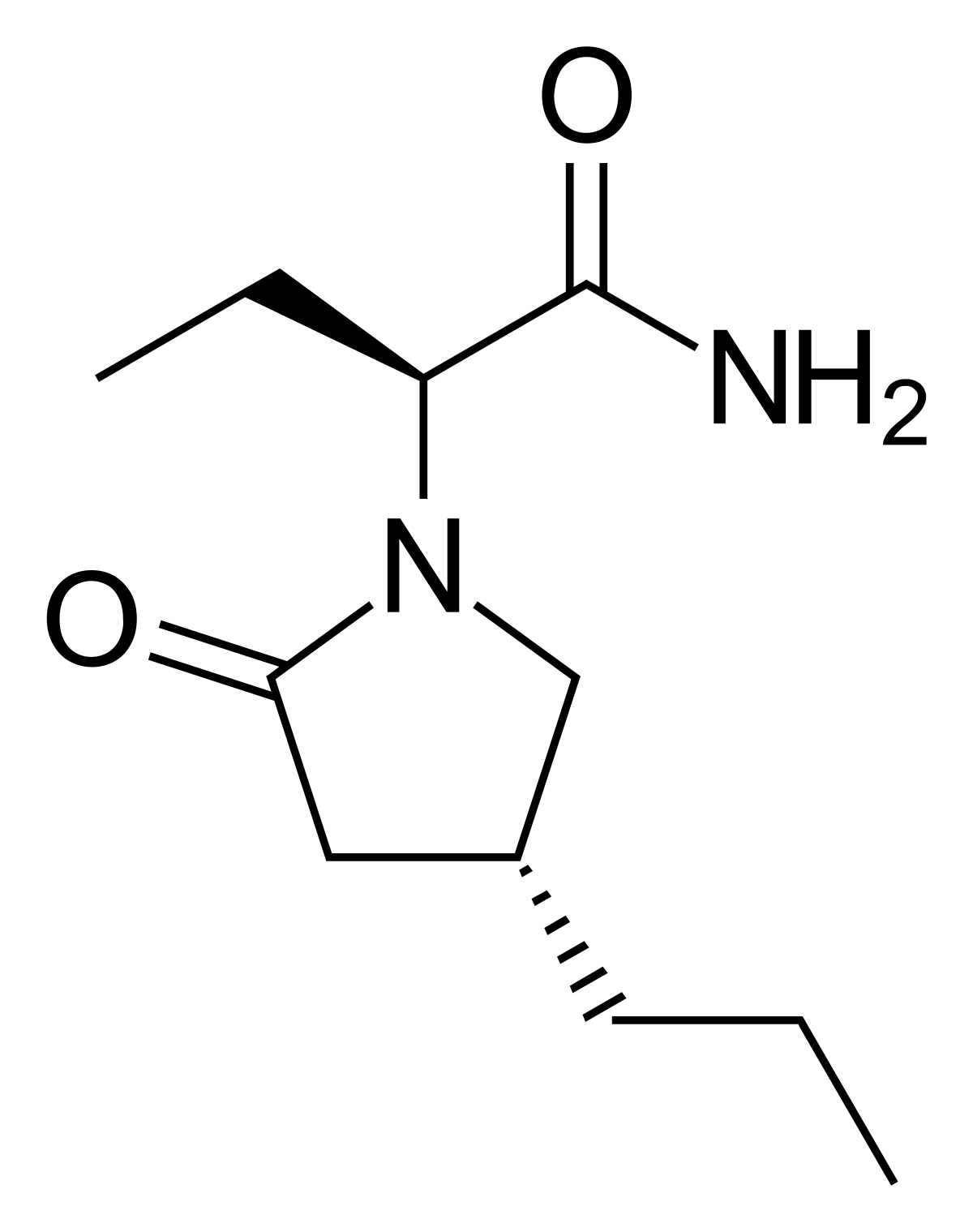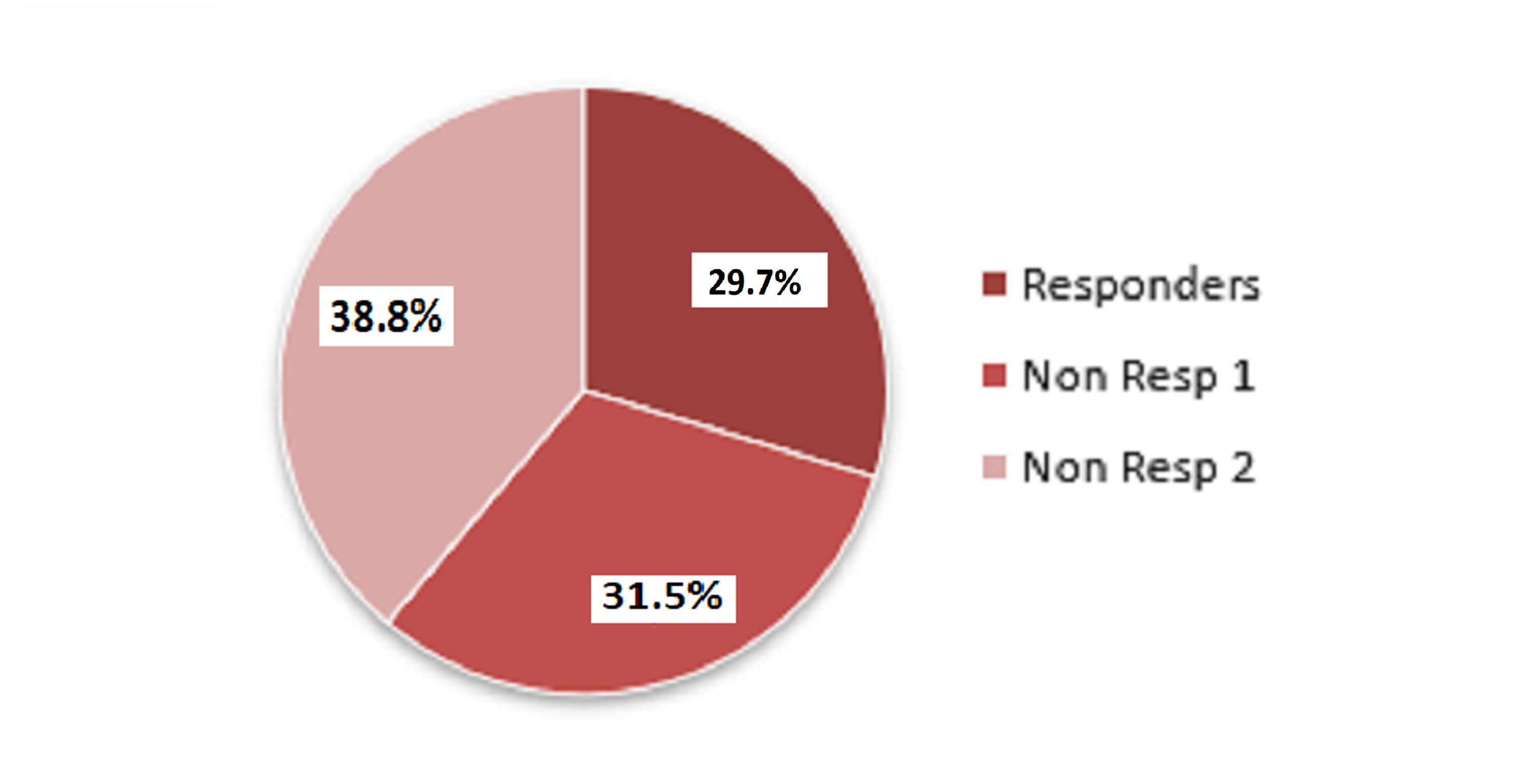
Cureus | Clinical and Electroencephalography Assessment of the Effects of Brivaracetam in the Treatment of Drug-Resistant Focal Epilepsy | Article
MEDICATION GUIDE BRIVIACT (briv ee akt) CV (brivaracetam) tablets, oral solution, and injection for intravenous use What is the

Favorable adverse effect profile of brivaracetam vs levetiracetam in a preclinical model - ScienceDirect

UCB Announces BRIVIACT® (Brivaracetam) now Approved by FDA to Treat Partial-onset (Focal) Seizures in Pediatric Epilepsy Patients - EpilepsyU

PDF) Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience
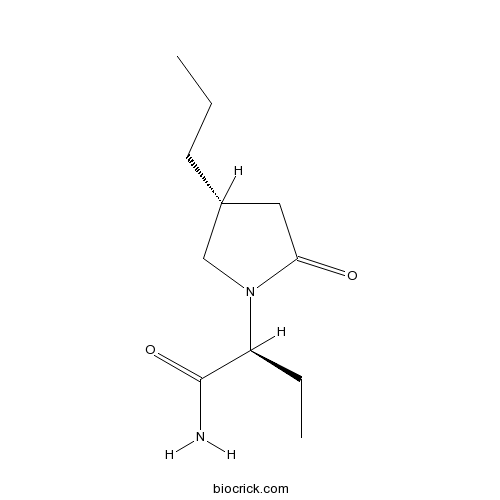
Brivaracetam | CAS:357336-20-0 | ligand for SV2A, selective and high-affinity | High Purity | Manufacturer BioCrick