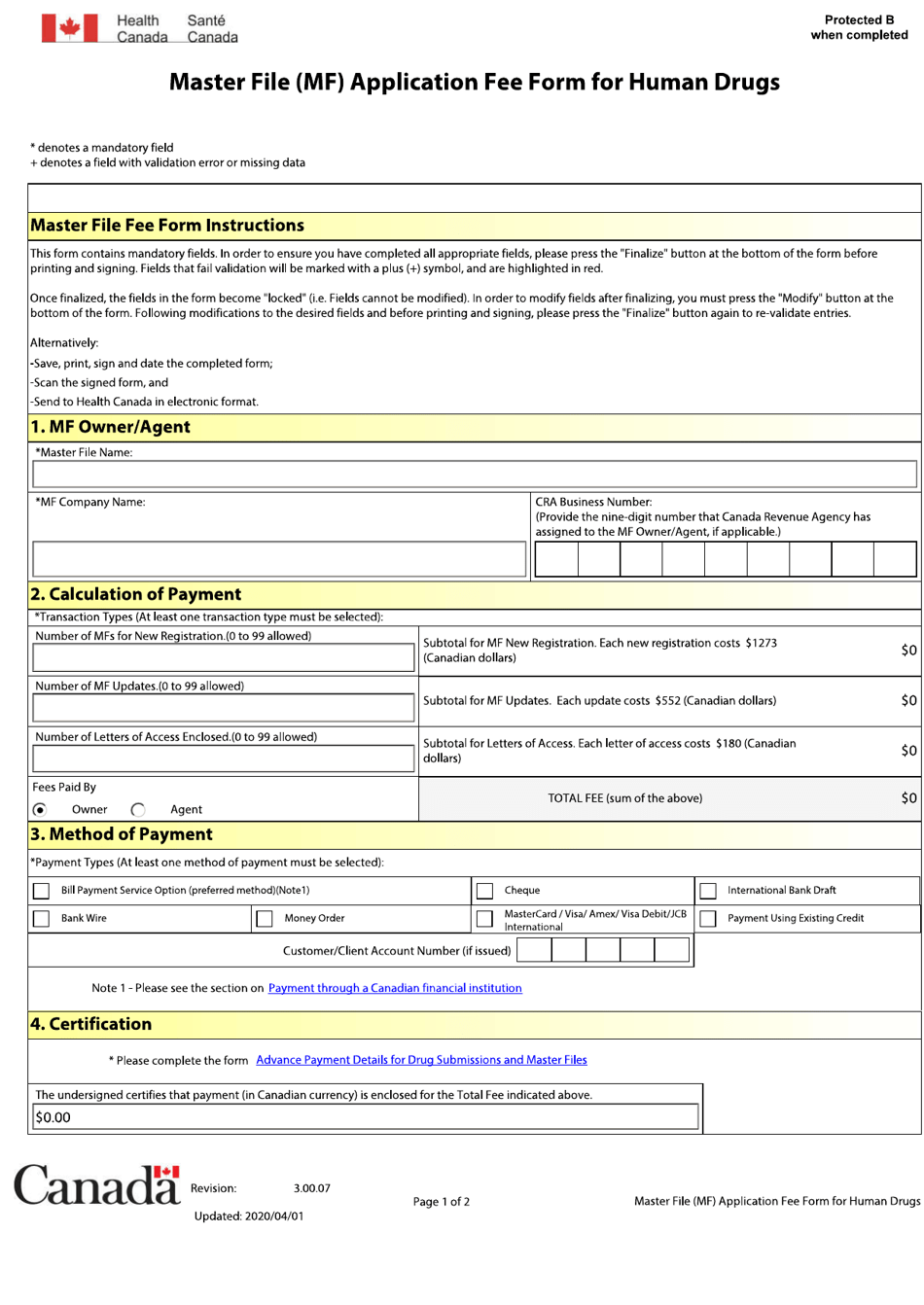
Fillable Online Health Canada CTO Registration Certificate - Wright Medical ... Fax Email Print - pdfFiller
Consultation: Registration of Clinical Trials and Public Disclosure of Results: Draft Guidance and Public Search Portal

Information Update - Health Canada advises Canadians about an unregistered pest control product on the market
BRIEF REPORT ON REGISTRATION GUIDELINES FOR NATURAL HEALTH PRODUCTS CANADA Country Canada Product Classification Natural He
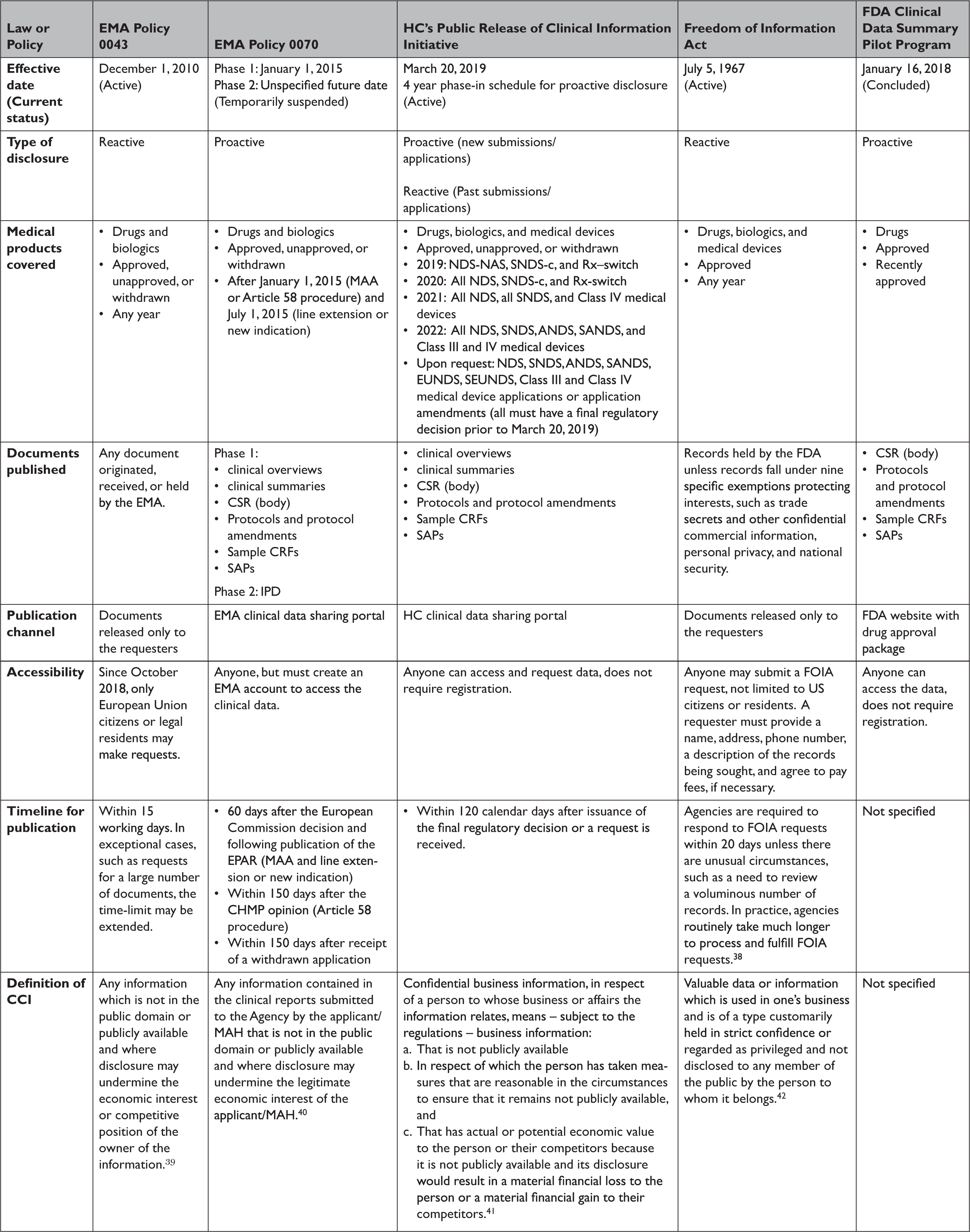
Transparency of Regulatory Data across the European Medicines Agency, Health Canada, and US Food and Drug Administration | Journal of Law, Medicine & Ethics | Cambridge Core

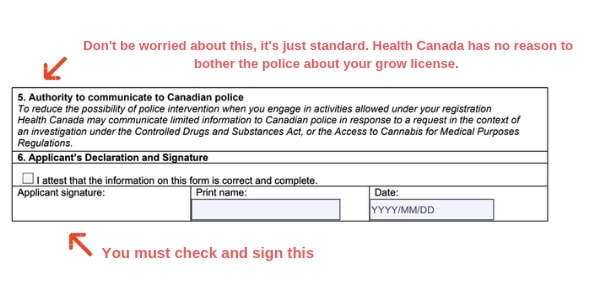
Health Canada - Access to Cannabis for Medical Purposes, Production for Own Medical / Production by a Designated Person : Cloud Practice


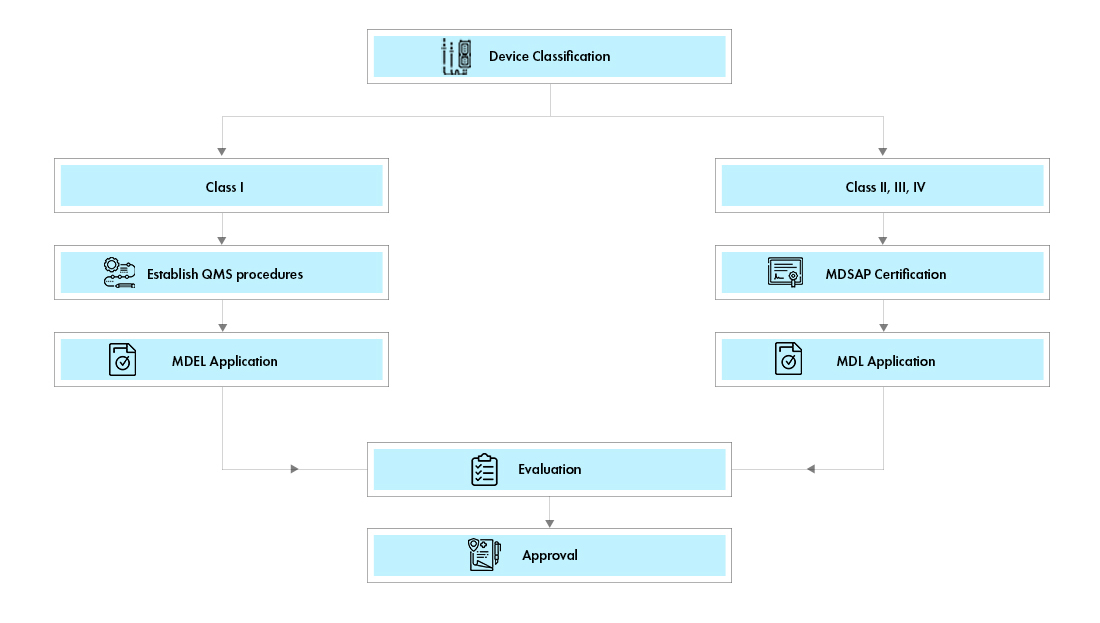



![Ottawa Chapter] Spotlight on Health Canada | science2business Ottawa Chapter] Spotlight on Health Canada | science2business](https://static.wixstatic.com/media/ddee1f_e46d5614dce443e78edb2ada1fdc5cd8~mv2.jpg/v1/fill/w_640,h_360,fp_0.50_0.50,q_80,usm_0.66_1.00_0.01,enc_auto/ddee1f_e46d5614dce443e78edb2ada1fdc5cd8~mv2.jpg)