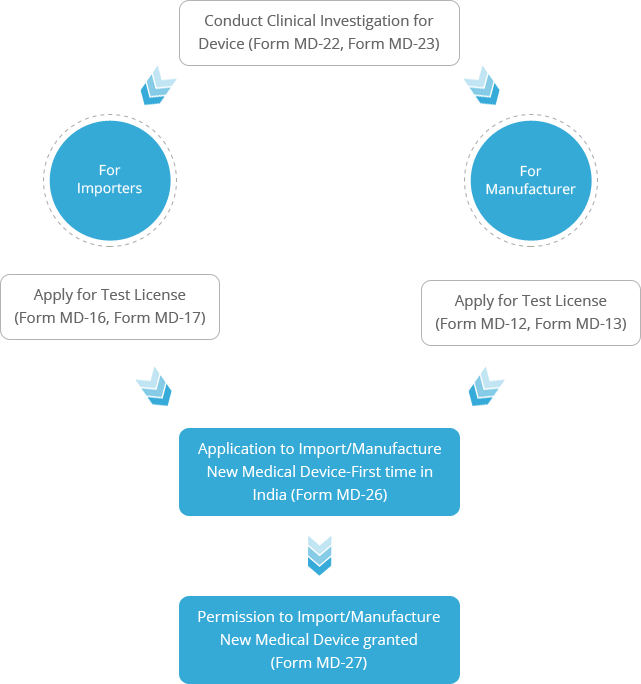
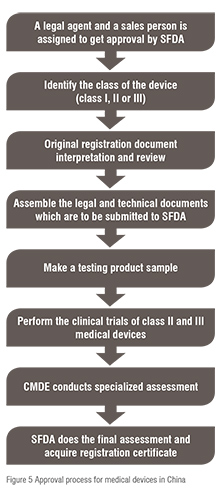
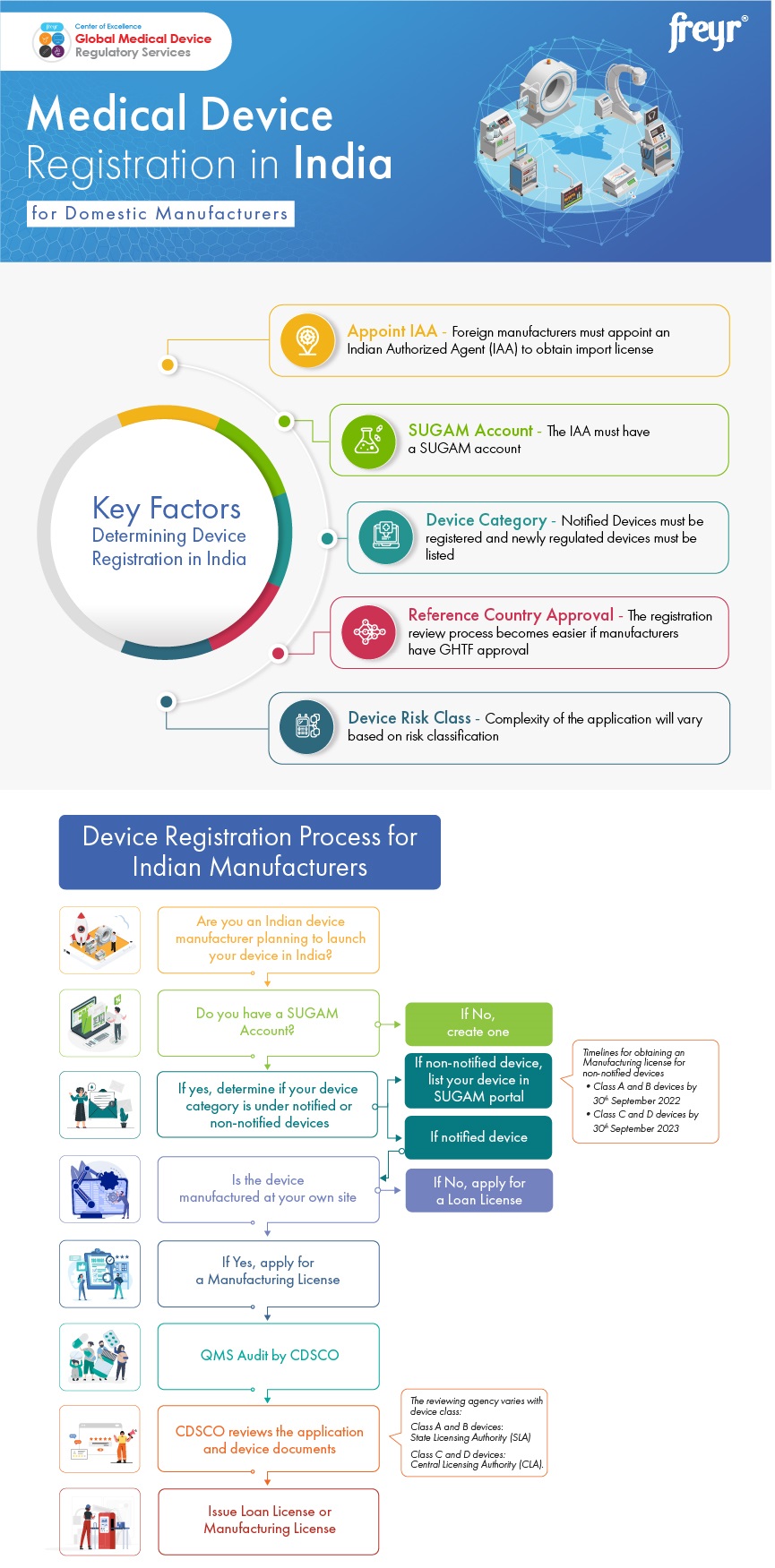
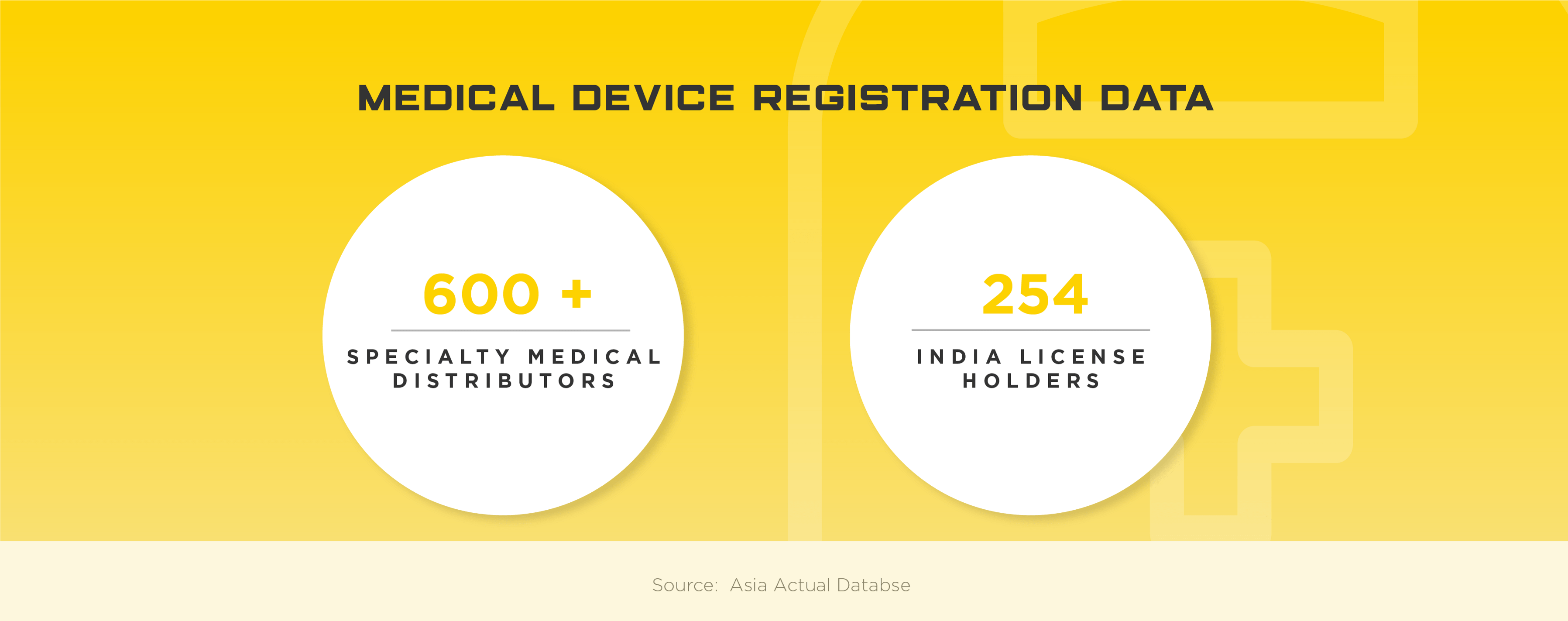
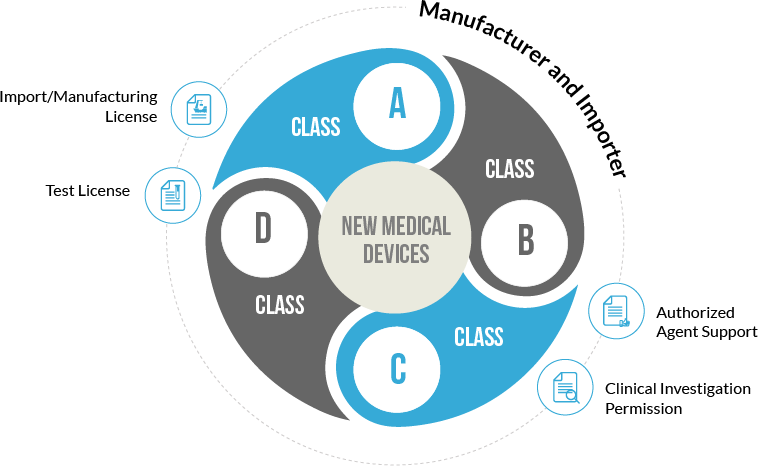
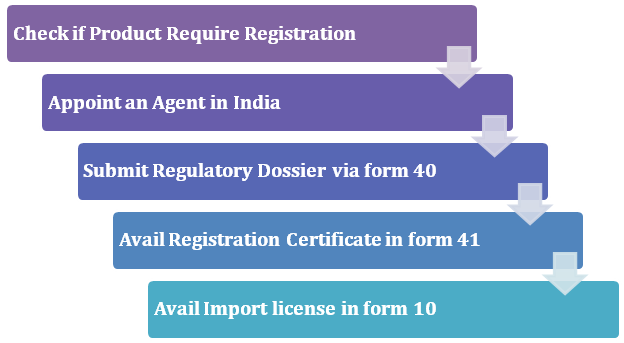
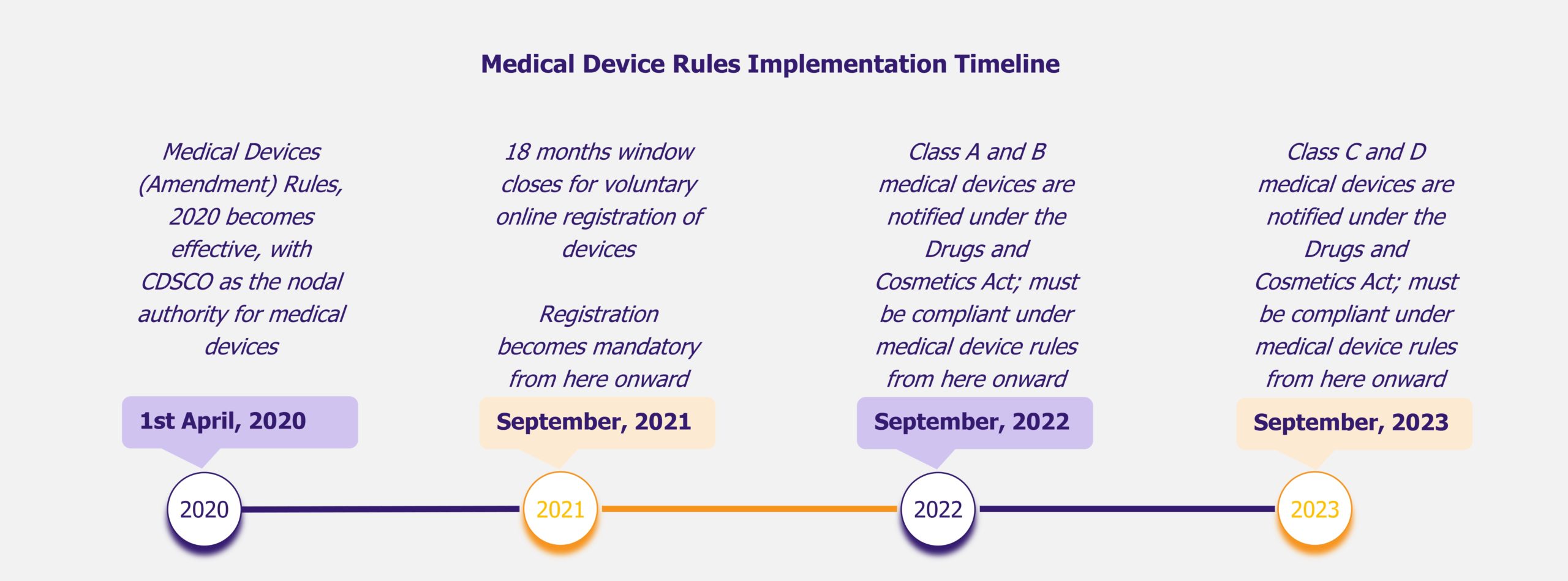
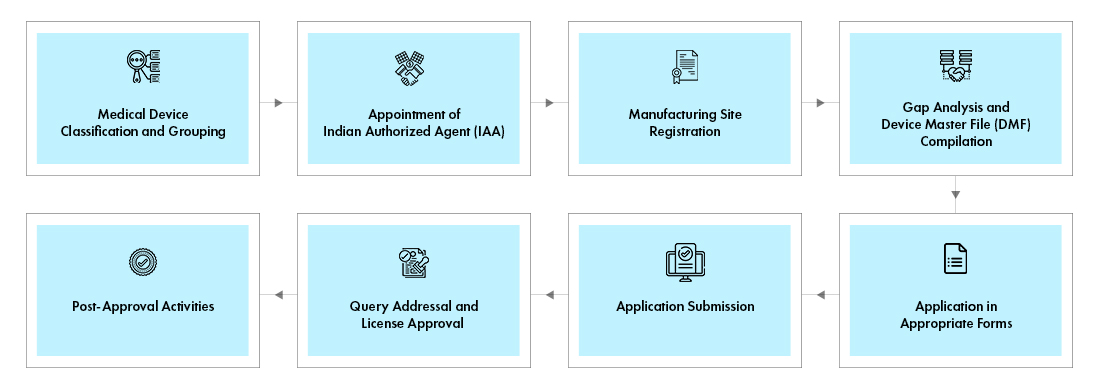
Mandatory Registration Scheme for Non-Notified Medical Device in India - Arazy Group Consultants Inc.

Frequently Asked Questions on new registration requirement for medical devices in India – Arogya Legal – The Health Laws Specialists
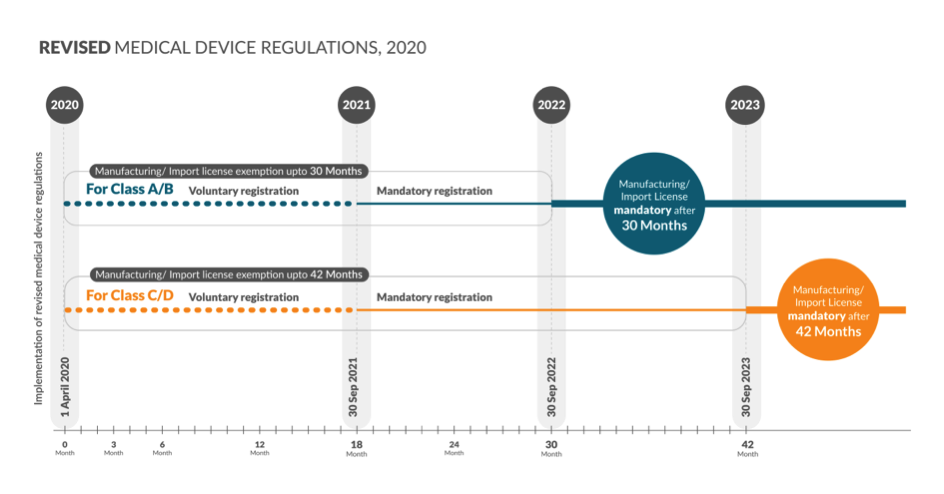
New Notification on Regulating All Devices – Deciphering the Key Concepts - CliniExperts -CliniExperts

Medical Device Registration: A Guide For Importers - Life Sciences, Biotechnology & Nanotechnology - India

Process of Registration Medical Devices in India | Guideline for License Approval: Monisha Chaudhary - YouTube